U.S. Food and Drug Administration (FDA)
FDA's role in antimicrobial resistance (AMR) preparedness and response, and information about AMR.
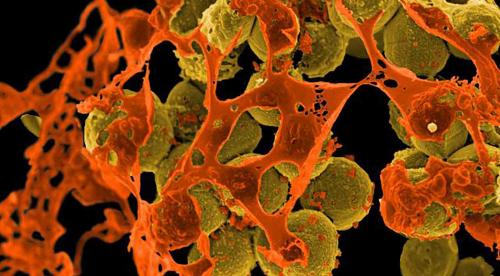
Scanning electron micrograph of methicillin-resistant Staphylococcus aureus (MRSA, brown) surrounded by cellular debris. MRSA resists treatment with many antibiotics. (Credit: NIAID)
Antimicrobial resistance (AMR)—the ability of a microorganism (bacteria, virus, fungi, parasite) to resist the effects of a drug—is a serious, complex and costly public health problem.
According to the Centers for Disease Control and Prevention (PDF, 148 pages), each year in the United States at least 2.8 million antibiotic-resistant infections occur, and more than 35,000 people die as a result. Combating AMR requires multifaceted efforts in both the healthcare and veterinary sectors.
What's new
- January 8, 2025: The FDA released a request for comments (RFC) soliciting public input on new opportunities and emergent monitoring needs for possible inclusion in the forthcoming National Antimicrobial Resistance Monitoring System (NARMS) 2026-2030 Strategic Plan. The Federal Register notice about the RFC includes instructions for submitting comments, as well as specific questions and requests for information meant to prompt helpful submissions. The 75-day comment period will be open from January 10 to March 26, 2025.
- November 18, 2024: New consumer article and video now available! Learn how you can safely use antibiotics so you can get well, protect yourself and your family, and combat antimicrobial resistance.
- October 10, 2024: The FDA announced the publication of the 2023 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. The 2023 data indicate that U.S. sales and distribution of medically important antimicrobial drugs approved for use in food-producing animals decreased by 2% between 2022 and 2023; this represents a 37% decrease in sales since the sales peaked in 2015.
- October 9, 2024: The U.S. Environmental Protection Agency (EPA) finalized its framework for expanding federal collaboration on the review of antibacterial and antifungal pesticides. In developing the framework, EPA coordinated with the U.S. Department of Health and Human Services (HHS) and the U.S. Department of Agriculture (USDA), under the oversight of the White House Office of Science and Technology Policy. The framework establishes a process for EPA to consider input from the other federal agencies on whether use of antibacterial or antifungal pesticides might reduce the effectiveness of some human and animal antibacterial and antifungal drugs.
The FDA's role and strategic approach
Antimicrobial resistance is recognized as a growing global threat. In 2014, the White House announced the National Strategy for Combating Antibiotic-Resistant Bacteria (CARB), underscoring the need for a coordinated inter-agency response to this threat. The FDA has been and continues to be integral in these efforts.
Several of FDA’s Centers—including the Center for Drug Evaluation and Research (CDER), Center for Devices and Radiological Health (CDRH), Center for Biologics Evaluation and Research (CBER), Center for Veterinary Medicine (CVM), National Center for Toxicological Research (NCTR), and the Office of the Chief Scientist—play key roles in combating AMR.
The FDA is dedicated to addressing the challenges AMR presents by helping to preserve the effectiveness of currently available antimicrobial drugs and promoting the development of new medical products that can help reduce the emergence and spread of AMR bacteria.
Working with both domestic and international partners, the FDA is proactively addressing the complex challenges associated with the growing threat of AMR by:
- Facilitating efficient product development to address AMR, including the development of new antimicrobials, diagnostic tests, and vaccines
- Promoting the appropriate and responsible use of antimicrobials and disseminating information promoting interventions that help slow the development of resistance
- Supporting the development and enhancement of tools for conducting surveillance of antimicrobial use and resistance so stakeholders can better track, treat, or respond to AMR outbreaks
- Advancing regulatory science to develop the tools, standards, and approaches to facilitate the translation of breakthrough discoveries in science and technology into innovative, safe, and effective medical products
To achieve this mission, the FDA will continue to work collaboratively with Congress, its partners at other U.S. government agencies, and other stakeholders to find additional ways to prevent, detect, and address AMR.
Product development
The FDA works closely with product sponsors and other government agencies to facilitate efficient product development to address AMR, including new antimicrobial drugs, biologics (including human vaccines), and diagnostics.
Employing product development program and incentives
Encouraging the development of novel and nontraditional products, and new tools
IVD devices for detection of AMR associated with microbial pathogens
Antimicrobial stewardship
The FDA works closely with domestic and international partners to promote the judicious use of antibiotics in the veterinary setting and complements the work done by other government agencies in the human healthcare setting.
Veterinary stewardship
Human healthcare stewardship
Surveillance and monitoring of antimicrobial use and resistance
The FDA works in close coordination with interagency partners and domestic stakeholders to collect the data necessary to conduct surveillance and monitoring of antimicrobial use and resistance.
Surveillance and monitoring activities and collaborations
Regulatory science
The FDA supports regulatory science to develop the tools, standards, and approaches to facilitate the translation of breakthrough discoveries in science and technology into innovative, safe, and effective medical products.
The 2022: Advancing Regulatory Science at FDA: Focus Areas of Regulatory Science (FARS) report outlines topics FDA has identified as needing continued targeted investment in regulatory science research to facilitate development of innovative products, provide data and methods to inform regulatory decision-making, and improve guidance to sponsors. Antimicrobial resistance is a priority area, under the focus area “Public Health Preparedness and Response.” Please contact FARS@fda.hhs.gov with questions about this initiative.
Tools for scientists
- CDC-FDA Antimicrobial Resistance Isolate Bank (AR Isolate Bank)
- Database for Reference Grade Microbial Sequences (FDA-ARGOS)
- FDA Launches Resistome Tracker, an Interactive Research and Data Visualization Tool for Antibiotic Resistance Genes
- National Database of Antibiotic Resistant Organisms (NDARO), from NIH
- NARMS Now: Human Data
- GenomeTrakr Network
- NARMS Now Integrated Data
Community stakeholders: We need your samples
As part of our effort to address the global health challenge of AMR, the FDA supports the development of next-generation sequencing (NGS)-based diagnostics to help healthcare providers identify and treat the right pathogen. To help build NGS infrastructure, our FDA-ARGOS database makes publicly available quality-controlled microbial reference genomes for diagnostic use. The FDA team is looking for unique, hard-to-source microbes like biothreat organisms, emerging pathogens, and AMR-related pathogens to help improve the database. We encourage the community to share microbe samplesExternal Link Disclaimer.
Select FDA research
Extramural funding opportunities
FDA publications
Guidance documents, compliance policy guides, and other FDA publications represent the FDA's current thinking on a topic. Documents related to antimicrobial resistance in humans and animals include guidances on developing products for treatment, prevention, and diagnosis of bacterial infections. You can also find information for the animal and veterinary industry on collection of sales and distribution of antimicrobial products, and veterinary feed directives.
You can search the entire database of FDA guidance documents by keyword, or visit the industry pages below for more information.
Drugs and biologics publications
Diagnostics and other device publications
Animal and veterinary publications
Information for consumers
FDA consumer updates
Know When and How to Use Antibiotics, and When to Skip ThemOther resources
Press and statements
- January 8, 2025: The FDA released a request for comments (RFC) soliciting public input on new opportunities and emergent monitoring needs for possible inclusion in the forthcoming National Antimicrobial Resistance Monitoring System (NARMS) 2026-2030 Strategic Plan. The Federal Register notice about the RFC includes instructions for submitting comments, as well as specific questions and requests for information meant to prompt helpful submissions. The 75-day comment period will be open from January 10 to March 26, 2025.
- October 10, 2024: The FDA announced the publication of the 2023 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. The 2023 data indicate that U.S. sales and distribution of medically important antimicrobial drugs approved for use in food-producing animals decreased by 2% between 2022 and 2023; this represents a 37% decrease in sales since the sales peaked in 2015.
- September 25, 2024: The FDA announced it is awarding funds for three projects to collect, analyze and report data on antimicrobial use (AMU) in animals. These projects support long term AMU data collection efforts under development within the U.S, including proposed public-private partnership frameworksExternal Link Disclaimer for tracking AMU data.
- July 15, 2024: The FDA authorized marketing of DiaSorin Molecular LLC’s Simplexa C. auris Direct, a molecular-based assay intended to detect Candida auris (C. auris) DNA from a skin swab of the armpit or groin from patients suspected of C. auris colonization. The test is intended to help prevent and control C. auris infections in health care settings. The assay may allow health care professionals to evaluate patients for colonization with C. auris faster than traditional culture-based techniques when such testing is needed. Faster detection can help stop the spread of this organism, which is frequently resistant to multiple antifungal drugs and can cause serious infections in hospitalized patients. Test results are meant to be used in conjunction with other clinical, epidemiologic, and laboratory information available to the clinician evaluating the patient. The test is not intended to diagnose or monitor treatment for C. auris infection. This is the latest example of the FDA’s ongoing commitment to helping ensure the development and expansion of tests for emerging infectious pathogens.
- June 21, 2024: The FDA converted Sirturo (bedaquiline) to traditional approval following a determination that a confirmatory trial verified clinical benefit. Sirturo is indicated for pulmonary tuberculosis (TB) due to Mycobacterium tuberculosis resistant to at least rifampin and isoniazid, also known as multi-drug resistant tuberculosis (MDR-TB), as part of a combination therapy, for adults and pediatric patients (5 years and older, weighing at least 15 kg). Sirturo was first approved in December 2012 under the FDA’s Accelerated Approval pathway. As part of the initial approval, the FDA required the applicant to conduct a confirmatory clinical study and develop a patient registry to assess rates of serious adverse events.
- April 24, 2024: FDA Approves New Treatment for Uncomplicated Urinary Tract Infections - The FDA approved Pivya (pivmecillinam) tablets for the treatment of female adults with uncomplicated urinary tract infections (UTIs) caused by susceptible isolates of Escherichia coli, Proteus mirabilis and Staphylococcus saprophyticus.
- April 9, 2024: FDA Approves New Antimicrobial Drug for Cattle and Swine - The FDA approved Pradalex (pradofloxacin injection) solution for certain respiratory diseases in cattle and swine. Pradofloxacin is a medically important antimicrobial in the fluoroquinolone class and may only be prescribed by a licensed veterinarian as a single injection. Over the past several decades, FDA has implemented policies to help ensure that medically important antimicrobials approved for use in animals are used in a manner that is consistent with principles of antimicrobial stewardship. For example, all medically important antimicrobials for animals require the authorization of a licensed veterinarian because FDA believes that, given their specialized training and experience, veterinarians play a critical role in antimicrobial stewardship and can help reduce the risks of antimicrobial resistance.
- April 3, 2024: The FDA approved Zevtera (ceftobiprole medocaril sodium for injection) for the treatment of adults with Staphylococcus aureus bloodstream infections (bacteremia) (SAB), including those with right-sided infective endocarditis; adults with acute bacterial skin and skin structure infections (ABSSSI); and adult and pediatric patients three months to less than 18 years old with community-acquired bacterial pneumonia (CABP). For more information, see the FDA press release: FDA Approves New Antibiotic for Three Different Uses.